Licensing deals struck by Chinese biotech start-ups with overseas partners could potentially fetch billions in revenue if their drug candidates are successfully developed and launched, according to analysts.
“One of the few highlights in the lacklustre sector was the strong deal flow from Chinese sellers to overseas buyers – mostly from the US – of preclinical or clinical-stage molecules, on so-called asset out-licensing or business development,” Zhang Jialin, head of China healthcare research, said in a report on January 21
New drugs typically take a decade to develop, and require preclinical research and animal testing to establish candidates’ safety and efficacy, before clinical trials on humans are conducted and applications are submitted to regulators for marketing approval.
Out-licensing typically involves a drug discoverer and patent owner selling the rights to another pharmaceutical company for further investment in clinical trials to bring them for regulatory approval and marketing in designated markets.
09:24
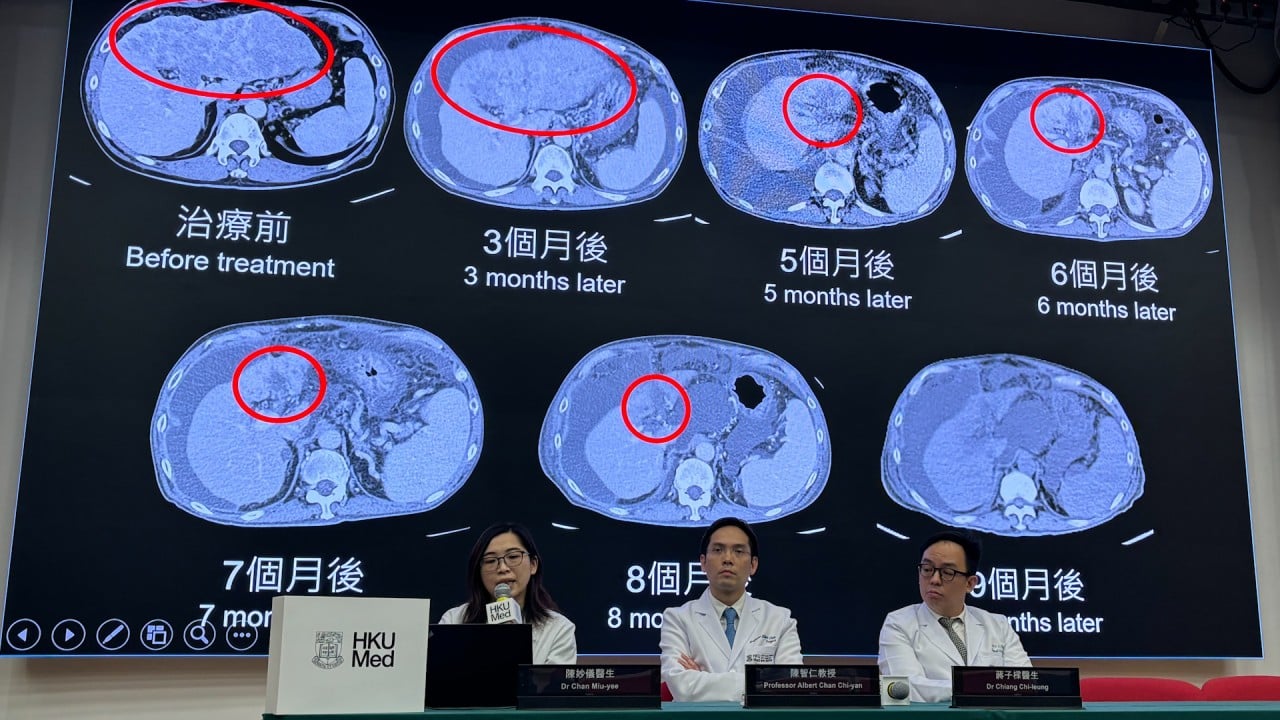
Cancer cure? Hong Kong develops ‘world’s first’ treatment to reverse stage-four liver cancer
Cancer cure? Hong Kong develops ‘world’s first’ treatment to reverse stage-four liver cancer
The MSCI China Health Care Index, which tracks 51 mid and large-capitalisation stocks, lost 19.5 per cent last year, trailing an 18.8 per cent gain in the broader MSCI China Index. The health sector gauge suffered losses ranging from 19 to 25 per cent in the last four years and underperformed the broader index in the past three years.

 By South China Morning Post | Created at 2025-01-31 09:09:52 | Updated at 2025-01-31 11:53:47
2 hours ago
By South China Morning Post | Created at 2025-01-31 09:09:52 | Updated at 2025-01-31 11:53:47
2 hours ago